Caribou Whispers Parity with Vispa-Cel. But Can Anyone Hear Them?
Caribou's optimized cohort matches YESCARTA/BREYANZI efficacy in LBCL, but $160M cash, no partner interest, and a too-narrow patient population make the commercial path treacherous
It’s been a while since my last post. I’ve been tied up working on a few new things (more on that in the future). But while I was off we had a slew of interesting oncology data readouts that I am catching up on, specifically at ESMO. I’ll have a dedicated ESMO post for subscribers soon, but for today I wanted to discuss the interested data update from Caribou Biosciences CRBU 0.00%↑ .
The company announced new data for its lead health donor allogeneic CD19 CAR-T program, vispa-cel (CB-010), in 2L+ relapsed/refractory (R/R) large B-cell lymphoma (LBCL). It also provided a minor update on its BCMA CAR-T program, but I will focus my comments on the CD19 update, given that was more interesting and material to the company’s future.
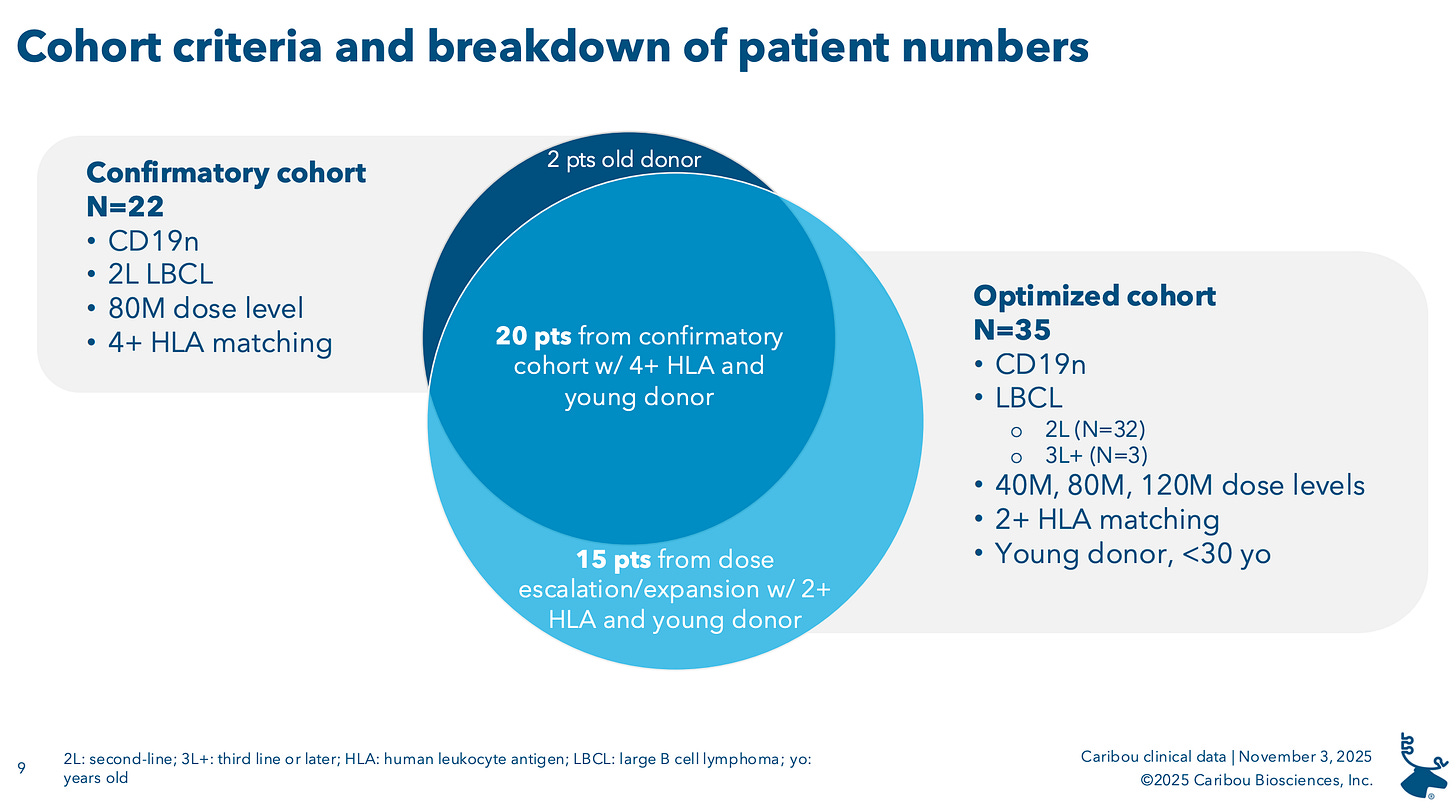
Recall, Caribou has had some ups and downs with this lead program, and last year pivoted to employing an HLA matching strategy in an effort to boost the persistence of its cells. This isn’t quite the full vision of what healthy-donor allo’ was supposed to be, where cell doses manufactured from any donor could go into any patient. But the realities of their data were that persistence was reducing the efficacy profile of its product, prompting them to tinker and deploy an HLA-matching strategy, similar to what Atara has tried to with Tab-Cel. It is still off-the-shelf treatment, but commercially, doctors would have the added step of HLA-typing their patients and checking with Caribou to confirm that that patient’s HLA-type is covered by donor pool. For reference, Caribou needs 10 batches to be available to cover 98% of LBCL patients. The previous 4+ HLA allele formula required 13 batches of product to cover 90% of the target patient population.
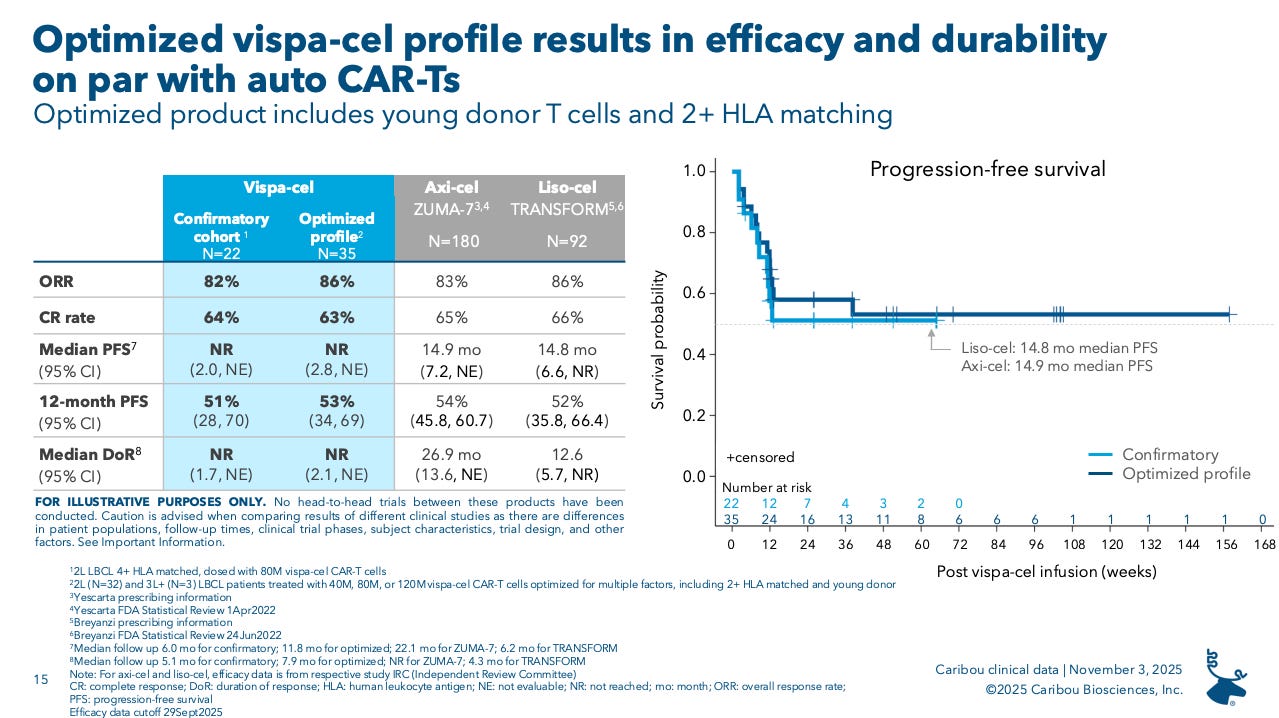
In this most recent update, Caribou reported data from the ‘optimized cohort’, encompassing patients who received product that matched two ore more HLA alleles and was derived from “young” donors (aka under 30 years old).
Side note: when I saw that they were qualifying “young” as under 30, I immediately felt old and washed. Sadly I am no longer “young” in the biotech sense. I am grizzled grey-bearded veteran. Aka “old”.
The data looked pretty solid in comparison to YESCARTA (axi-cel) and BREYANZI (liso-cel), the two leading CD19 CAR-Ts in this space, with comparable efficacy albeit in a much smaller patient population. A profile that is on par with current CAR-Ts but with the convenience of an off-the-shelf approach, could tip the scales and enable more patients to choose CAR-T as a 2L option. Right now that isn’t happening all that much (25% according to Caribou), due to limited/no availability at community hospitals. Caribou is hoping a safety profile with low G3+ CRS and ICANS can penetrate into this Community hospitals, where physicians are typically using non-cell therapy treatment options and waiting to refer-out patients for CAR-T in the third line setting.
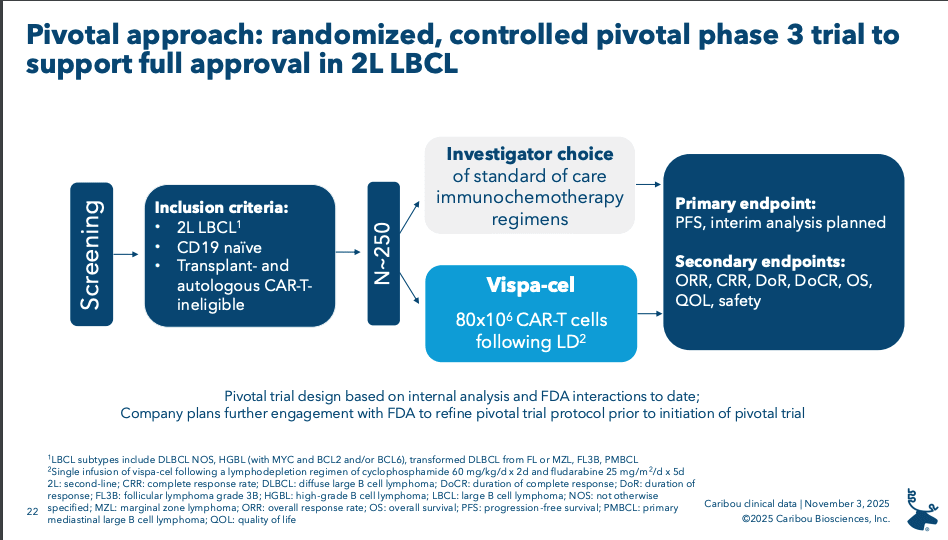
Next up for Caribou is determining whether this 2+ HLA matching strategy can work in a prospective fashion, followed by a P3 study in ASCT and CAR-T ineligible 2L patients.
Shares were up nearly 180% into the market open and roughly 30% when trading open but then came back down to earthy with the company ending the day flat. That tells me investors liked the headline numbers, but post investor call some harsh realities about the path ahead set in. It is unclear exactly what those are, but I have some ideas on what they might be.